Mesenchymal Stem Cell - Derived EVs (MSC-EVs)
Mesenchymal Stem Cell-derived EVs
With the current breakthroughs in scientific and clinical research into Mesenchymal Stem Cell EVs, the opportunity has arisen for the development of high-impact biopharmaceuticals for the delivery of therapeutic molecules to our body’s cells.
Pre-clinical and underway clinical studies indicate that the Data-Rich Cargo of MSC EV’s (the proteins, nucleic acids, bioactive lipids, metabolites, and mitochondria) within MSC EV’s can improve the function of aging, injured or diseased cells, tissues and organs.
As an ideal vehicle for delivery, the lipid membrane of EVs serves not only to protect proteins and RNA from degradation but also allows EVs to reach cells in parts of the body that many drugs cannot. EVs are able to cross the blood-brain barrier and also penetrate solid tissue masses.
The mode of action of mesenchymal stem cell extracellular vesicles (MSC-EVs) is that they exert their effects through various mechanisms such as delivering bioactive molecules, modulating gene expression, and interacting with target cells. MSC-EVs are known to contain a variety of biomolecules such as microRNAs, proteins, and lipids, which can regulate various cellular processes such as apoptosis, angiogenesis, and inflammation. When MSC-EVs are taken up by target cells, these biomolecules can modulate cellular signaling pathways and gene expression to promote tissue repair, reduce inflammation, and modulate the immune response.
Umbilical Cord Mesenchymal Stem Cell Extracellular Vesicles (UCMSC-EVs)
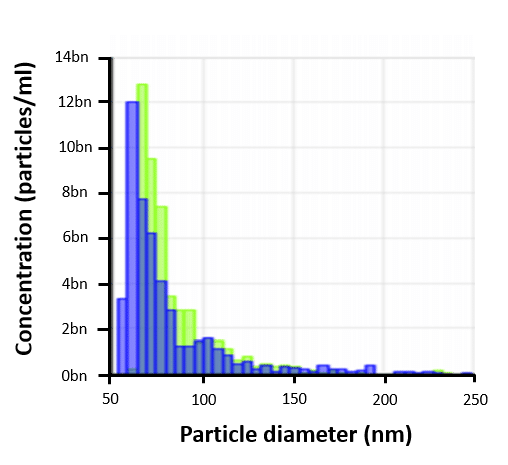
UCMSC-EV Size Distribution
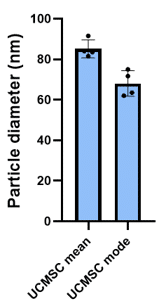
UCMSC-EV Mean and Mode Diameter
UCMSC-EVs have a particle size distribution mainly below 100nm, indicating enrichment of highly therapeutic small Extracellular Vesicles (EVs), including exosomes.
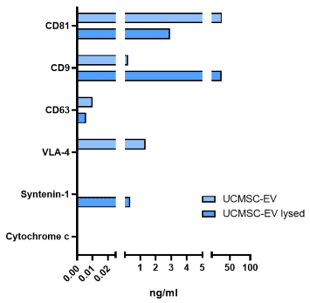
EV Marker Expression
Components
EVs are known to contain diverse therapeutic molecules including:
Immunomodulatory molecules:
TSG-6, miR‐574‐5p, PGE2, IL-10, IDO, MHC
Growth factors:
HGF, TGFB, EGF, BDNF, VEGF, FGF
Cytoprotective factors:
HSP60/70/90, Annexin A1, miR-21, miR-126, Catalase
UCMSC-EVs are confirmed to express five positive markers, including four surface proteins CD81, CD9, CD63, and VLA-4, and one cytosolic protein, Syntentin-1 contained within EVs. The purified EVs were clear of the negative cellular marker Cytochrome-c.
SC21 MSC-EV Bioactivity and Skin Regeneration Function
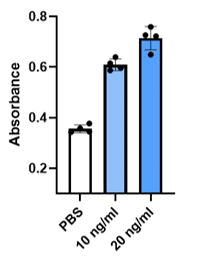
Energy Metabolism and Protein Synthesis
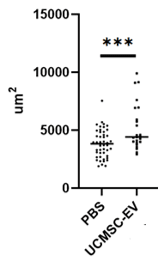
Cell Size
Wound Healing
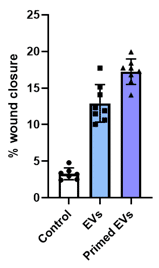
SC21 EVs are rigorously tested for bioactivity. EVs were tested for their potential in skin regeneration. When added to dermal fibroblasts the EVs stimulated a dose-dependent increase in energy use and protein synthesis (A) (important early steps in regeneration), and the target fibroblasts increased in size (B), which is another sign of activation of the cells for repair or regeneration. Finally, as seen in (C), EVs had a potent effect impact on wound healing.
SC21 MSC-EV Stimulate Skin Fibroblasts to Produce Regenerative Factors
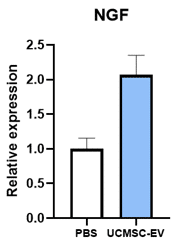
Nerve Growth Factor
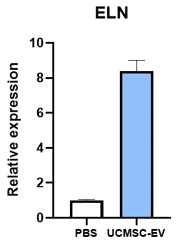
Elastin
Collagen
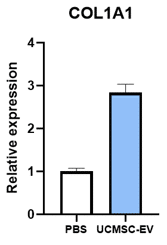
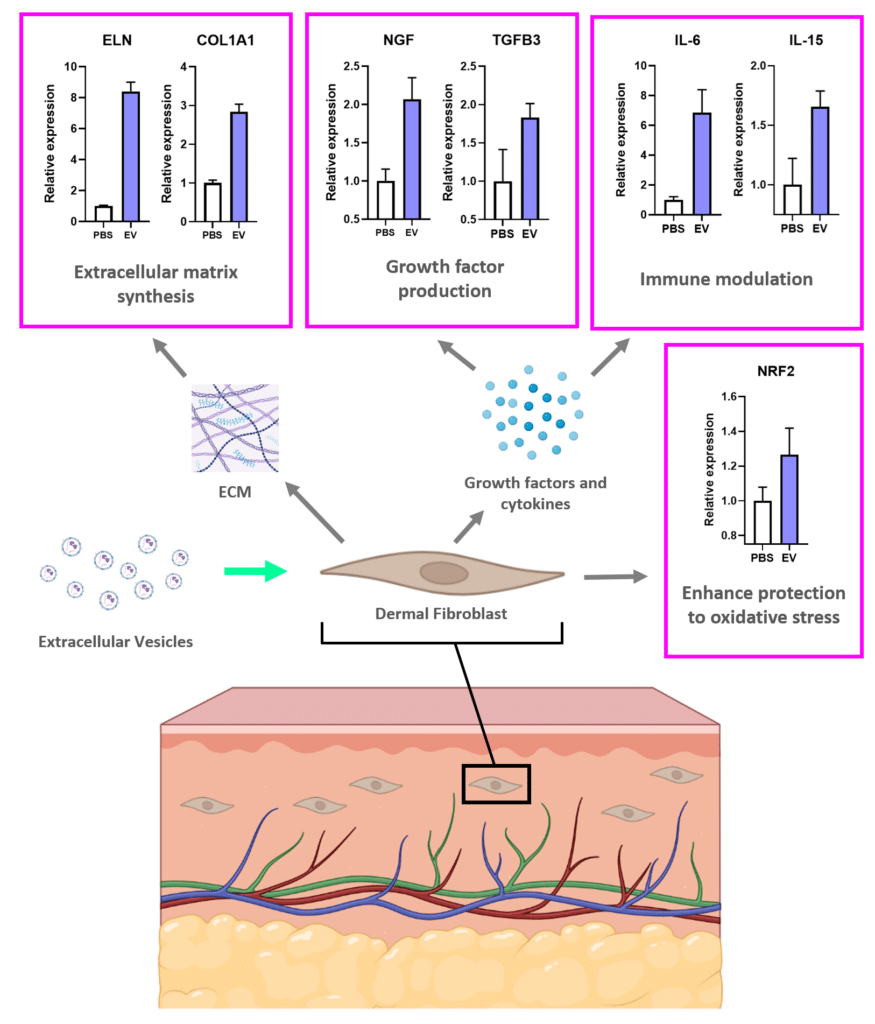
Adding EVs to dermal fibroblasts stimulates them to increase production of Collagen (COL1A1) and Elastin (ELN), two of the most important compounds of young and healthy skin. The addition of EVs also causes the production of nerve growth factor (NGF) which plays an important role in skin regeneration and can regenerate and support the health of peripheral nerves. SC21 EVs were also shown cause an increase in skin cell expression of other genes that can contribute to wound healing or skin regeneration, including IL-6, IL-15, TGFB3, and NRF2.
Functional Impact of MSC-EVs for Skin Regeneration
SC21 EVs have applications in multiple disease. Their ability to suppress harmful inflammation, protect cells from Reactive Oxygen Species (ROS) or DNA damage, and stimulate cells to repair and regenerate. One application SC21 has studied extensively is the potential of EVs for skin rejuvenation and regeneration. Genetic analysis of fibroblasts after EV application showed activation of multiple skin regenerative systems including synthesis of Extracellular Matrix (A), production of growth factors (B), modulation of reparative immune activity (C), and enhanced protection to oxidative damage (D)